

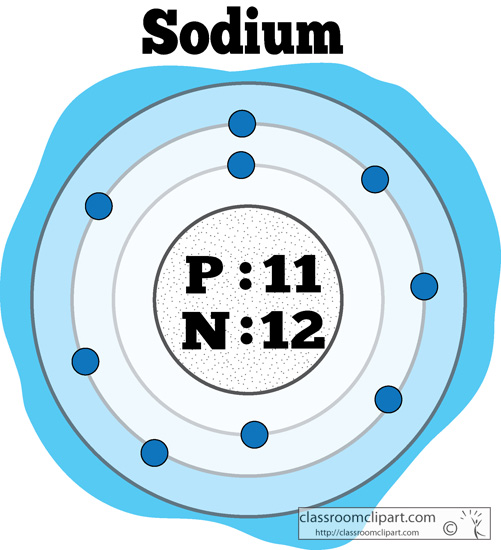
Sodium usually forms ionic compounds involving the Na+ cation. Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. In the periodic table, the elements are listed in order of increasing atomic number Z.Įlectron configuration of Sodium is 3s1. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. Therefore, the number of electrons in neutral atom of Sodium is 11. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
#Sodium atom full
Therefore its activity accounts for most of the reactor coolants activity when reactor is at full power. It is radioactive and created from common sodium-23 by neutron bombardment. Sodium-24 is one of the most important isotopes. Sodium-24 is composed of 11 protons, 12 neutrons, and 11 electrons. It is also commonly used as a positron source in positron annihilation spectroscopy. odium-22 is a radioactive isotope of sodium, undergoing positron emission to 22Ne with a half-life of 2.605 years.

Sodium-22 is composed of 11 protons, 11 neutrons, and 11 electrons. By measuring the concentration of this isotope, the neutron radiation dosage to the victim can be computed. Acute neutron radiation exposure (e.g., from a nuclear criticality accident) converts some of the stable 23Na in human blood plasma to 24 Sodium-23 is composed of 11 protons, 12 neutrons, and 11 electrons. Two radioactive, cosmogenic isotopes are the byproduct of cosmic ray spallation: 22Na has a half-life of 2.6 years and 24Na, a half-life of 15 hours all other isotopes have a half-life of less than one minute. Mass numbers of typical isotopes of Sodium are 23. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. © Keith S.Sodium is a chemical element with atomic number 11 which means there are 11 protons in its nucleus. Please use the search box to find pages / postings on specific themes. She was interviewed near the start of her college 'A level'. Annie was a participant in the Understanding Chemical Bonding project.


 0 kommentar(er)
0 kommentar(er)
